

In Part 4, participants will receive durvalumab 1120 mg (15 mg/kg) + bevacizumab 15 mg/kg every 3 weeks (Q3W).
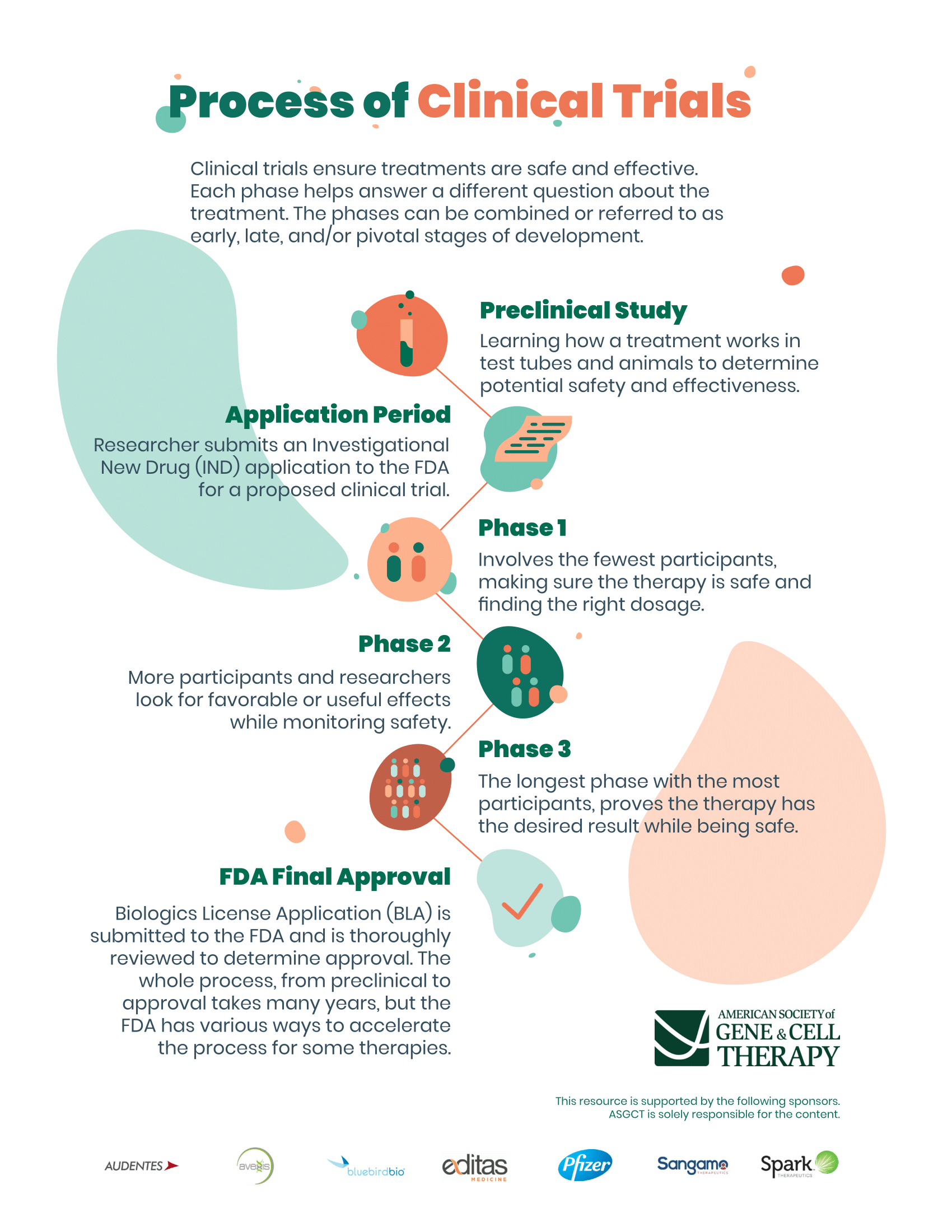
Participants will be randomized at a ratio of 2:1:2 in 'Durvalumab 1500 mg Q4W', 'Tremelimumab 750 mg Q4W for 7 doses followed by Q12W', and 'Tremelimumab 300 mg × 1 dose + durvalumab 1500 mg Q4W' arms, respectively. Tremelimumab 75 mg Q4W × 4 doses + durvalumab 1500 mg Q4W, followed by durvalumab 1500 mg Q4W.įollowing protocol amendment 5, enrollment into 'Tremelimumab 75 mg Q4W × 4 doses + durvalumab 1500 mg' arm will close.Tremelimumab 750 mg Q4W for 7 doses followed by Q12W.Tremelimumab 300 mg × 1 dose + durvalumab 1500 mg Q4W.In Part 3, participants will be randomized in a 2:2:1:2 ratio to receive: In Part 2B, participants will receive tremelimumab 300 mg × 1 dose + durvalumab 1500 mg Q4W.In China cohort, Part 2A study design will be followed.Tremelimumab 1 mg/kg Q4W × 4 doses + durvalumab 20 mg/kg Q4W, followed by durvalumab 20 mg/kg Q4W.Tremelimumab 10 mg/kg Q4W × 7 doses followed by every 12 weeks (Q12W).
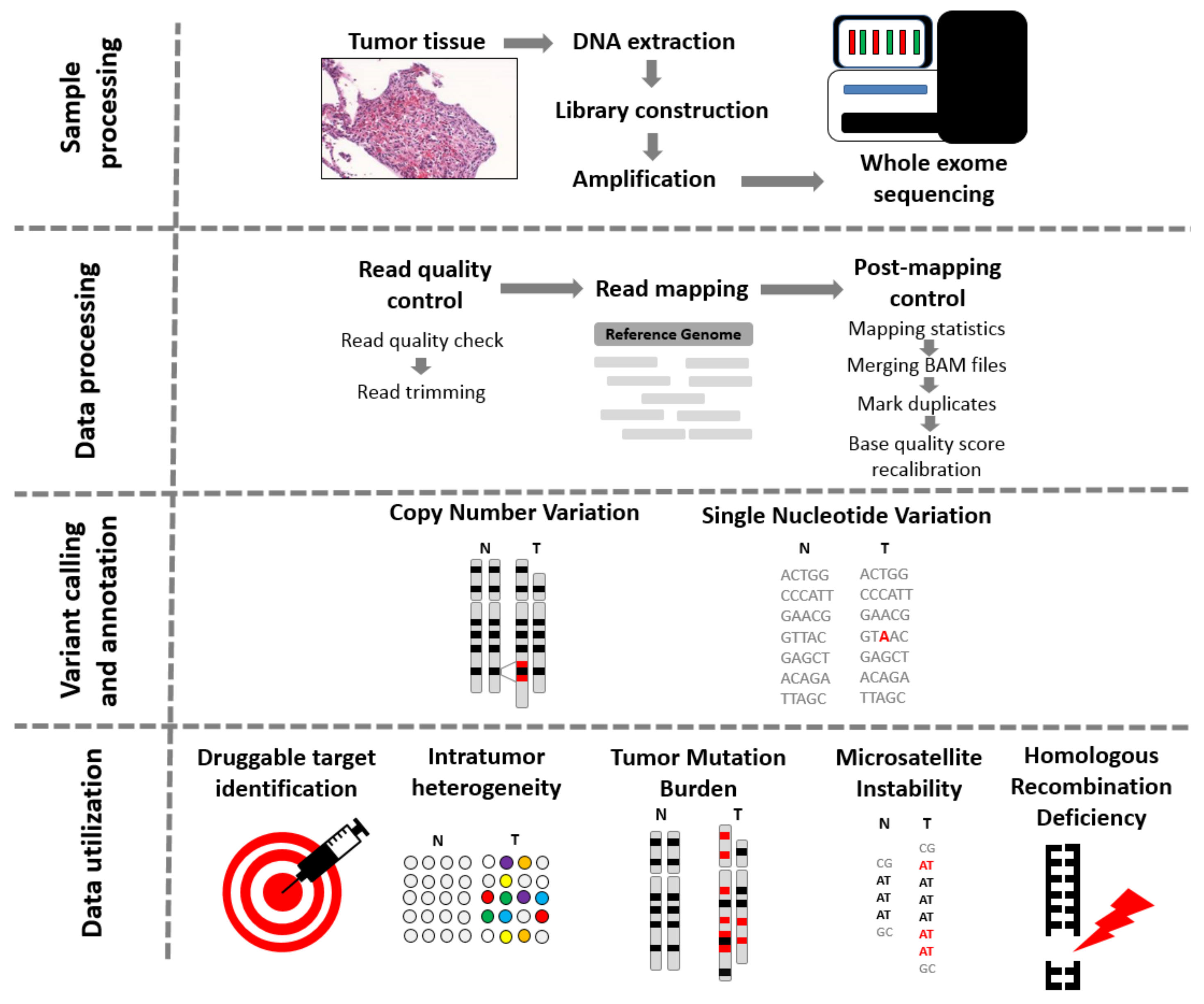
In Part 2A, participants will be randomized in a 1:1:1 ratio to receive:


 0 kommentar(er)
0 kommentar(er)
